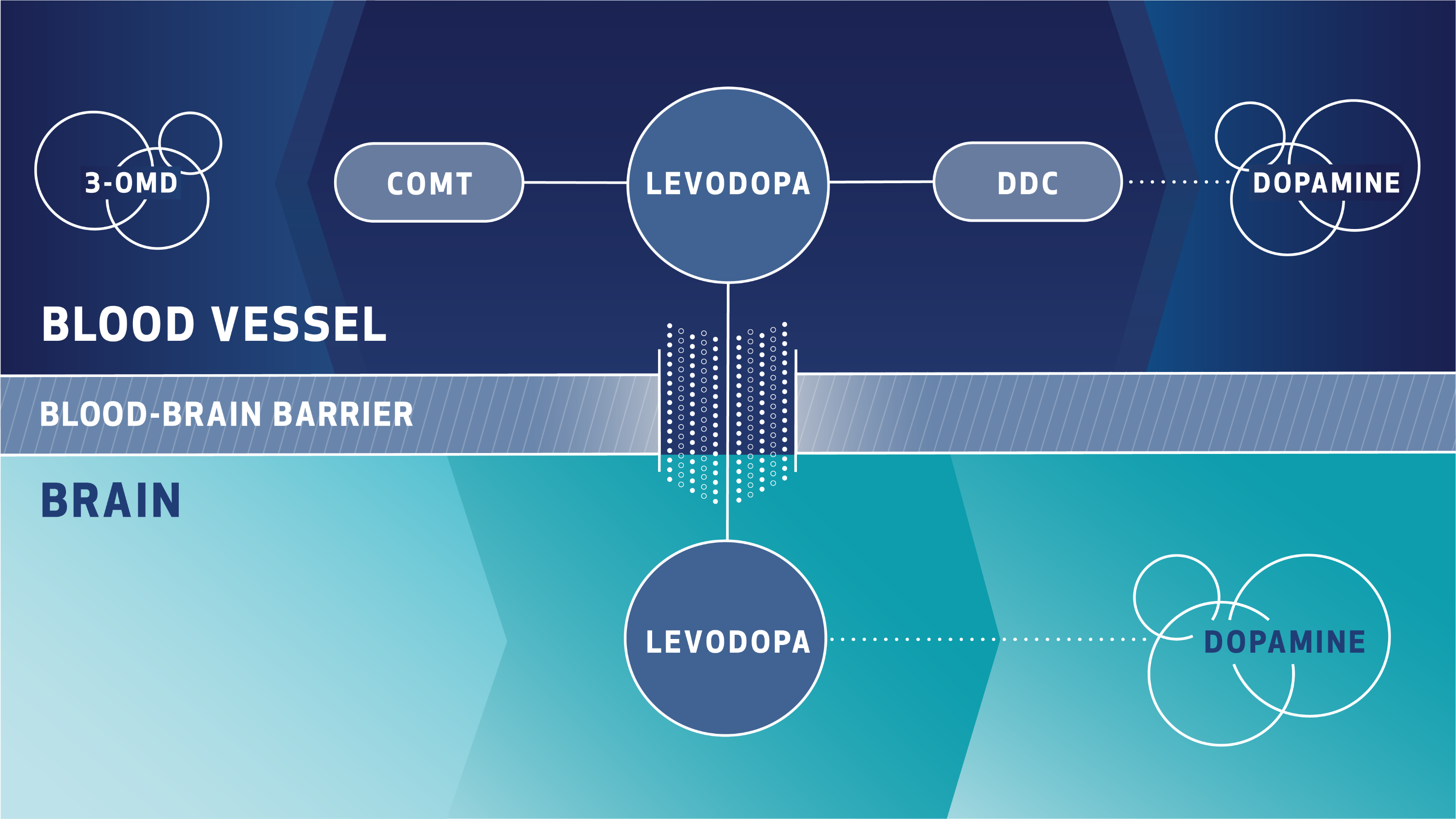
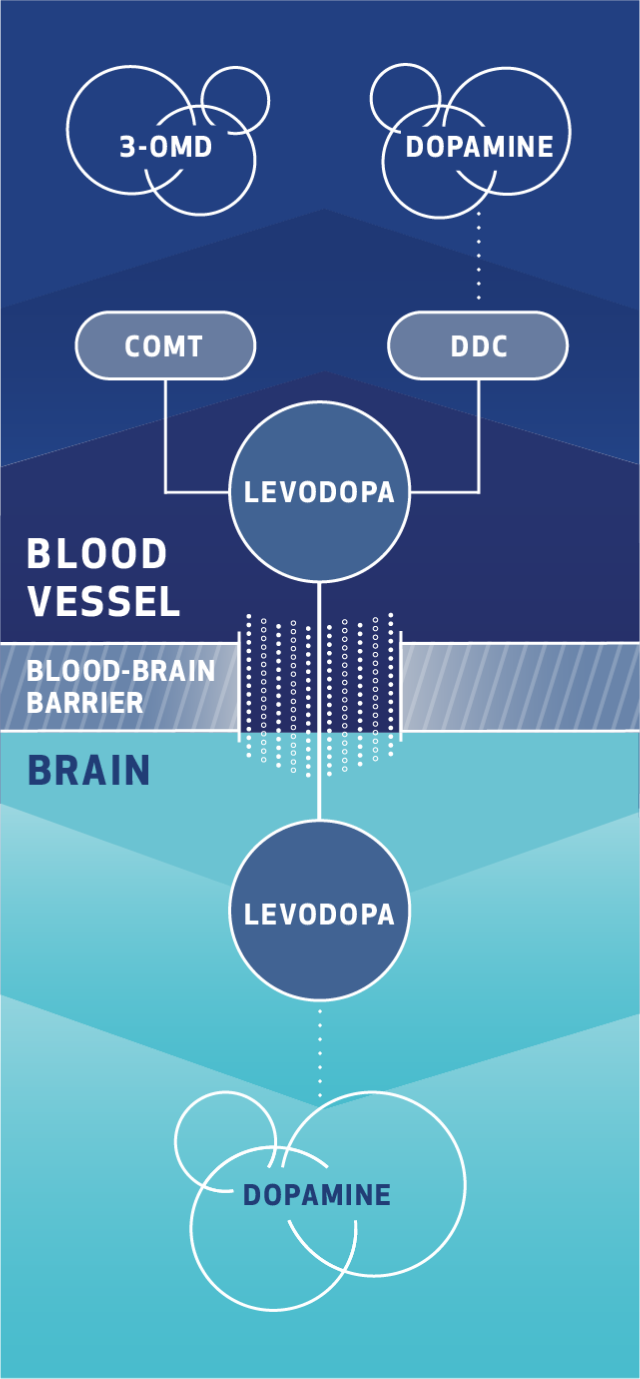
Cardiovascular Effects with Concomitant Use of Drugs Metabolized by Catechol-O-Methyltransferase (COMT) - Possible arrhythmias, increased heart rate, and excessive changes in blood pressure may occur with concomitant use of ONGENTYS and drugs metabolized by COMT, regardless of the route of administration (including inhalation). Monitor patients treated concomitantly with ONGENTYS and drugs metabolized by COMT.
Falling Asleep During Activities of Daily Living and Somnolence - Patients have reported falling asleep while engaged in activities of daily living, including driving, which may result in accidents. Consider discontinuing ONGENTYS or adjusting other dopaminergic/sedating medications. Advise patients to avoid driving and other potentially dangerous activities.
Hypotension/Syncope - Monitor patients for hypotension and advise patients about the risk for syncope. If necessary, consider discontinuing ONGENTYS or adjusting the dosage of other medications that can lower blood pressure.
Dyskinesia - ONGENTYS may cause or exacerbate dyskinesia. Consider levodopa or dopaminergic medication dose reduction.
Hallucinations and Psychosis - Consider stopping ONGENTYS if these occur. Patients with a major psychotic disorder should ordinarily not be treated with ONGENTYS.
Impulse Control/Compulsive Disorders - Patients may experience intense urges (eg, gambling, sexual, spending money, binge eating) and the inability to control them. It is important for prescribers to ask about the development of new or increased urges. Monitor for occurrence of intense urges and consider discontinuing ONGENTYS if they occur.
Withdrawal-Emergent Hyperpyrexia and Confusion - A symptom complex resembling neuroleptic malignant syndrome can develop with rapid dose reduction or withdrawal of drugs that increase central dopaminergic tone. When discontinuing ONGENTYS, monitor patients and consider adjustment of dopaminergic therapies as needed.
The most common adverse reactions (incidence at least 4% and greater than placebo) were dyskinesia, constipation, blood creatine kinase increased, hypotension/syncope, and weight decreased.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit MedWatch at or call .
Please see ONGENTYS full .
References:
- Goodall M, Alton H. Metabolism of 3,4-dihydroxyphenylalanine (L-dopa) in human subjects. Biochem Pharmacol. 1972;21(17):2401-2408.
- Männistö PT, Kaakkola S. Catechol-O-methyltransferase (COMT): biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacol Rev. 1999;51(4):593-628.
- Reilly DK, Rivera-Calimlim L, Van Dyke D. Catechol-O-methyltransferase activity: a determinant of levodopa response. Clin Pharmacol Ther. 1980;28(2):278-286.
- Cedarbaum JM. Clinical pharmacokinetics of antiparkinsonian drugs. Clin Pharmacokinet. 1987;13(3):141-178.
- Andersson I, Granerus AK, Jagenburg R, Svanborg A. Intestinal decarboxylation of orally administered L-dopa: influence of pharmacological preparation, dose magnitude, dose sequence and food intake. Acta Med Scand. 1975;198(5):415-420.
- Kuruma I, Bartholini G, Tissot R, Pletscher A. Comparative investigation of inhibitors of extracerebral dopa decarboxylase in man and rats. J Pharm Pharmacol. 1972;24(4):289-294.
- Reilly DK, Rivera-Calimlim L. Red blood cell catechol-O-methyl transferase, plasma 3-O-methyldopa and dyskinesias [abstract 57]. Pharmacologist. 1978;20:156.
- Connolly BS, Lang AE. Pharmacological treatment of Parkinson disease: a review. JAMA. 2014;311(16):1670-1683.
- Rivera-Calimlim L, Tandon D, Anderson F, Joynt R. The clinical picture and plasma levodopa metabolite profile of parkinsonian nonresponders: treatment with levodopa and decarboxylase inhibitor. Arch Neurol. 1977;34(4):228-232.
- Dingemanse J, Kleinbloesem CH, Zürcher G, Wood ND, Crevoisier C. Pharmacodynamics of benserazide assessed by its effects on endogenous and exogenous levodopa pharmacokinetics. Br J Clin Pharmacol. 1997;44(1):41-48.
- Wade LA, Katzman R. 3-O-methyldopa uptake and inhibition of L-dopa at the blood-brain barrier. Life Sci. 1975;17(1):131-136.
- Fabbri M, Rosa MM, Ferreira JJ. Adjunctive therapies in Parkinson's disease: how to choose the best treatment strategy approach. Drugs Aging. 2018;35(12):1041-1054.
- Ondo WG. Motor complications in Parkinson's disease. Int J Neurosci. 2011;121(suppl 2):37-44.
- Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson's disease (2009). Neurology. 2009;72(21)(suppl 4):S1-S136.
- ONGENTYS [package insert]. Bridgewater, NJ: Amneal Pharmaceuticals LLC; 2023.
- Data on file. Amneal Pharmaceuticals LLC.
- Rocha J-F, Falcão A, Santos A, et al. Effect of opicapone and entacapone upon levodopa pharmacokinetics during three daily levodopa administrations. Eur J Clin Pharmacol. 2014;70(9):1059-1071.
- Rocha JF, Ebersbach G, Lees A, et al. The added benefit of opicapone when used in Parkinson's disease patients with levodopa-induced motor fluctuations: a post-hoc analysis of BIPARK-I and -II. Front Neurol. 2021;12:754016.
- Dézsi L, Vécsei L. Monoamine oxidase B inhibitors in Parkinson's disease. CNS Neurol Discord Drug Targets. 2017;16(4):425-439.
- Stocchi F, Torti M, Fossati C. Advances in dopamine receptor agonists for the treatment of Parkinson's disease. Expert Opin Pharmacother. 2016;17(14):1889-1902.